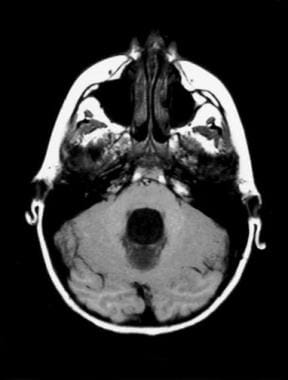
Hydrocephalus can be defined broadly as a disturbance of cerebrospinal fluid (CSF) formation, flow, or absorption, leading to an increase in volume occupied by this fluid in the central nervous system (CNS).
[1] This condition could also be termed a hydrodynamic CSF disorder. See the image below.
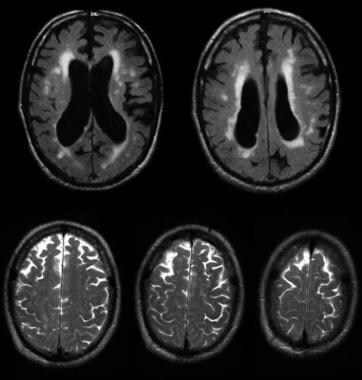
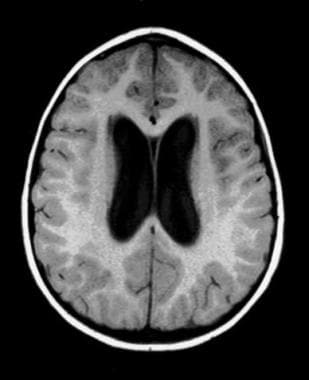
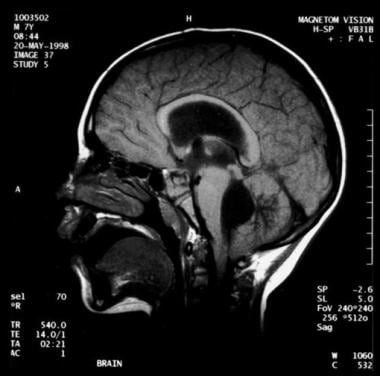 Noncommunicating obstructive hydrocephalus caused by obstruction of the foramina of Luschka and Magendie. This MRI sagittal image demonstrates dilatation of lateral ventricles with stretching of corpus callosum and dilatation of the fourth ventricle.
Noncommunicating obstructive hydrocephalus caused by obstruction of the foramina of Luschka and Magendie. This MRI sagittal image demonstrates dilatation of lateral ventricles with stretching of corpus callosum and dilatation of the fourth ventricle.
Signs and symptoms
Clinical features of hydrocephalus are influenced by the patient's age, the cause of the hydrocephalus, the location of the obstruction, its duration, and its rapidity of onset.
Symptoms in infants include poor feeding, irritability, reduced activity, and vomiting.
Symptoms in children and adults include the following:
Slowing of mental capacity, cognitive deterioration
Headaches (initially in the morning)
Neck pain, suggesting tonsillar herniation
Vomiting, more significant in the morning
Blurred vision: A consequence of papilledema and, later, of optic atrophy
Double vision: Related to unilateral or bilateral sixth nerve palsy
Difficulty in walking secondary to spasticity: Preferentially affects the lower limbs because the periventricular pyramidal tract is stretched by the hydrocephalus
Drowsiness
Children may also exhibit stunted growth and sexual maturation from third ventricle dilatation. Adults may also have nausea that is not exacerbated by head movements; incontinence (urinary first, fecal later if condition remains untreated) indicates significant destruction of the frontal lobes and advanced disease.
Symptoms of normal pressure hydrocephalus (NPH) include the following:
Gait disturbance: Usually the first symptom and may precede other symptoms by months or years; magnetic gait is used to emphasize the tendency of the feet to remain "stuck to the floor" despite patients’ best efforts to move them
Dementia (of varying degrees): Should be a late finding in pure (shunt-responsive) NPH; presents as an impairment of recent memory or as a "slowing of thinking"; spontaneity and initiative are decreased
Urinary incontinence: May present as urgency, frequency, or a diminished awareness of the need to urinate
Other symptoms that can occur: Personality changes and Parkinsonism
Rarely: Headaches; seizures are extremely rare—consider an alternative diagnosis
Diagnosis
Examination in infants may reveal the following findings:
Head enlargement (head circumference ≥98th percentile for age)
Dysjunction of sutures
Dilated scalp veins
Tense fontanelle
Setting-sun sign: Characteristic of increased intracranial pressure (ICP); downward deviation of ocular globes, retracted upper lids, visible white sclerae above iris
Increased limb tone (spasticity preferentially affects the lower limbs)
Children and adults may demonstrate the following findings on physical examination:
Papilledema
Failure of upward gaze: Due to pressure on the tectal plate through the suprapineal recess; the limitation of upward gaze is of supranuclear origin
Unsteady gait
Large head
Unilateral or bilateral sixth nerve palsy (secondary to increased ICP)
Children may also exhibit the Macewen sign, in which a "cracked pot" sound is noted on percussion of the head.
Patients with NPH may exhibit the following findings on examination:
Normal muscle strength; no sensory loss
Increased reflexes and Babinski response in one or both feet: Search for vascular risk factors (causing associated brain microangiopathy or vascular Parkinsonism), which are common in NPH patients
Variable difficulty in walking: May have mild imbalance to inability to walk or to stand; the classic gait impairment consists of short steps, wide base, externally rotated feet, and lack of festination (hastening of cadence with progressively shortening stride length, a hallmark of the gait impairment of
Parkinson disease)
Frontal release signs (in late stages): Appearance of sucking and grasping reflexes
Testing
No specific blood tests are recommended in the workup for hydrocephalus. However, consider genetic testing and counseling when X-linked hydrocephalus is suspected, and evaluate the CSF in posthemorrhagic and postmeningitic hydrocephalus for protein concentration and to exclude residual infection.
Obtain electroencephalography in patients with seizures.
Imaging studies
The following imaging studies may be used to evaluate patients with suspected hydrocephalus:
Computed tomography (CT) scanning: To assess size of ventricles and other structures
Magnetic resonance imaging (MRI): To assess for Chiari malformation or cerebellar or periaqueductal tumors
Ultrasonography through anterior fontanelle in infants: To assess for subependymal and intraventricular hemorrhage; to follow infants for possible progressive hydrocephalus
Skull radiography: To detect erosion of sella turcica, or "beaten copper cranium" (or "beaten silver cranium")—the latter can also be seen in craniosynostosis; (after shunt insertion) to confirm correct positioning of installed hardware
MRI cine: To measure CSF stroke volume (SV) in the cerebral aqueduct; however, such measurements don’t appear to be useful in predicting response to shunting
[2]
Diffusion tensor imaging (DTI): To detect differences in fractional anisotropy and mean diffusivity of the brain parenchyma surrounding the ventricles; allows recognition of microstructural changes in periventricular white matter region that may be too subtle on conventional MRI
[3]
Radionuclide cisternography (in NPH): To assess the prognosis with regard to possible shunting—however, due to its poor sensitivity in predicting shunt response when the ventricular to total intracranial activity (V/T) ratio is less than 32%, this test is no longer commonly used
Management
Surgery
Surgical treatment is the preferred therapeutic option in patients with hydrocephalus.
[4] Most patients eventually undergo shunt placements, such as the following:
Ventriculoperitoneal (VP) shunt (most common)
Ventriculoatrial (VA) shunt (or "vascular shunt")
Lumboperitoneal shunt: Only used for communicating hydrocephalus, CSF fistula, or pseudotumor cerebri)
Torkildsen shunt (rarely): Effective only in acquired obstructive hydrocephalus
Ventriculopleural shunt (second-line therapy): Used if other shunt types contraindicated
Rapid-onset hydrocephalus with ICP is an emergency. The following procedures can be done, depending on each specific case:
Ventricular tap in infants
Open ventricular drainage in children and adults
Lumbar puncture (LP) in posthemorrhagic and postmeningitic hydrocephalus
VP or VA shunt
Repeat LPs can be performed for cases of hydrocephalus after intraventricular hemorrhage (which can resolve spontaneously). If reabsorption does not resume when the CSF protein content is less than 100 mg/dL, spontaneous resorption is unlikely to occur. LPs can be performed only in cases of communicating hydrocephalus.
Alternatives to shunting include the following:
Choroid plexectomy or choroid plexus coagulation
Opening of a stenosed aqueduct
Endoscopic fenestration of the floor of the third ventricle (however, contraindicated in communicating hydrocephalus)
Conservative management
Medical treatment is not effective in long-term treatment of chronic hydrocephalus; it is used as a temporizing measure to delay surgical intervention. Medical therapy may be tried in premature infants with posthemorrhagic hydrocephalus (in the absence of acute hydrocephalus). Normal CSF absorption may resume spontaneously during this interim period.
Medication as treatment for hydrocephalus is controversial and should be used only as a temporary measure for posthemorrhagic hydrocephalus in neonates. Such agents include carbonic anhydrase inhibitors (eg, acetazolamide) and loop diuretics (eg, furosemide).