Practice Essentials
Hydrocephalus can be defined broadly as a disturbance of cerebrospinal fluid (CSF) formation, flow, or absorption, leading to an increase in volume occupied by this fluid in the central nervous system (CNS).[1] This condition could also be termed a hydrodynamic CSF disorder. See the image below.
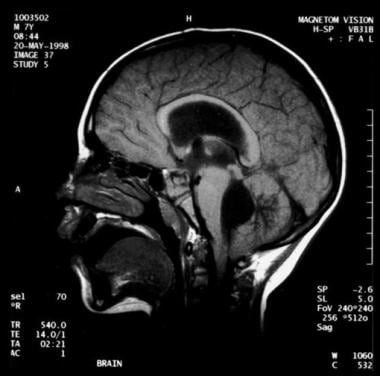 Noncommunicating obstructive hydrocephalus caused by obstruction of the foramina of Luschka and Magendie. This MRI sagittal image demonstrates dilatation of lateral ventricles with stretching of corpus callosum and dilatation of the fourth ventricle.
Noncommunicating obstructive hydrocephalus caused by obstruction of the foramina of Luschka and Magendie. This MRI sagittal image demonstrates dilatation of lateral ventricles with stretching of corpus callosum and dilatation of the fourth ventricle.Signs and symptoms
Clinical features of hydrocephalus are influenced by the patient's age, the cause of the hydrocephalus, the location of the obstruction, its duration, and its rapidity of onset.
Symptoms in infants include poor feeding, irritability, reduced activity, and vomiting.
Symptoms in children and adults include the following:
- Slowing of mental capacity, cognitive deterioration
- Headaches (initially in the morning)
- Neck pain, suggesting tonsillar herniation
- Vomiting, more significant in the morning
- Blurred vision: A consequence of papilledema and, later, of optic atrophy
- Double vision: Related to unilateral or bilateral sixth nerve palsy
- Difficulty in walking secondary to spasticity: Preferentially affects the lower limbs because the periventricular pyramidal tract is stretched by the hydrocephalus
- Drowsiness
Children may also exhibit stunted growth and sexual maturation from third ventricle dilatation. Adults may also have nausea that is not exacerbated by head movements; incontinence (urinary first, fecal later if condition remains untreated) indicates significant destruction of the frontal lobes and advanced disease.
Symptoms of normal pressure hydrocephalus (NPH) include the following:
- Gait disturbance: Usually the first symptom and may precede other symptoms by months or years; magnetic gait is used to emphasize the tendency of the feet to remain "stuck to the floor" despite patients’ best efforts to move them
- Dementia (of varying degrees): Should be a late finding in pure (shunt-responsive) NPH; presents as an impairment of recent memory or as a "slowing of thinking"; spontaneity and initiative are decreased
- Urinary incontinence: May present as urgency, frequency, or a diminished awareness of the need to urinate
- Other symptoms that can occur: Personality changes and Parkinsonism
- Rarely: Headaches; seizures are extremely rare—consider an alternative diagnosis
See Clinical Presentation for more detail.
Diagnosis
Examination in infants may reveal the following findings:
- Head enlargement (head circumference ≥98th percentile for age)
- Dysjunction of sutures
- Dilated scalp veins
- Tense fontanelle
- Setting-sun sign: Characteristic of increased intracranial pressure (ICP); downward deviation of ocular globes, retracted upper lids, visible white sclerae above iris
- Increased limb tone (spasticity preferentially affects the lower limbs)
Children and adults may demonstrate the following findings on physical examination:
- Papilledema
- Failure of upward gaze: Due to pressure on the tectal plate through the suprapineal recess; the limitation of upward gaze is of supranuclear origin
- Unsteady gait
- Large head
- Unilateral or bilateral sixth nerve palsy (secondary to increased ICP)
Children may also exhibit the Macewen sign, in which a "cracked pot" sound is noted on percussion of the head.
Patients with NPH may exhibit the following findings on examination:
- Normal muscle strength; no sensory loss
- Increased reflexes and Babinski response in one or both feet: Search for vascular risk factors (causing associated brain microangiopathy or vascular Parkinsonism), which are common in NPH patients
- Variable difficulty in walking: May have mild imbalance to inability to walk or to stand; the classic gait impairment consists of short steps, wide base, externally rotated feet, and lack of festination (hastening of cadence with progressively shortening stride length, a hallmark of the gait impairment ofParkinson disease)
- Frontal release signs (in late stages): Appearance of sucking and grasping reflexes
Testing
No specific blood tests are recommended in the workup for hydrocephalus. However, consider genetic testing and counseling when X-linked hydrocephalus is suspected, and evaluate the CSF in posthemorrhagic and postmeningitic hydrocephalus for protein concentration and to exclude residual infection.
Obtain electroencephalography in patients with seizures.
Imaging studies
The following imaging studies may be used to evaluate patients with suspected hydrocephalus:
- Computed tomography (CT) scanning: To assess size of ventricles and other structures
- Magnetic resonance imaging (MRI): To assess for Chiari malformation or cerebellar or periaqueductal tumors
- Ultrasonography through anterior fontanelle in infants: To assess for subependymal and intraventricular hemorrhage; to follow infants for possible progressive hydrocephalus
- Skull radiography: To detect erosion of sella turcica, or "beaten copper cranium" (or "beaten silver cranium")—the latter can also be seen in craniosynostosis; (after shunt insertion) to confirm correct positioning of installed hardware
- MRI cine: To measure CSF stroke volume (SV) in the cerebral aqueduct; however, such measurements don’t appear to be useful in predicting response to shunting [2]
- Diffusion tensor imaging (DTI): To detect differences in fractional anisotropy and mean diffusivity of the brain parenchyma surrounding the ventricles; allows recognition of microstructural changes in periventricular white matter region that may be too subtle on conventional MRI [3]
- Radionuclide cisternography (in NPH): To assess the prognosis with regard to possible shunting—however, due to its poor sensitivity in predicting shunt response when the ventricular to total intracranial activity (V/T) ratio is less than 32%, this test is no longer commonly used
See Workup for more detail.
Management
Surgery
Surgical treatment is the preferred therapeutic option in patients with hydrocephalus.[4] Most patients eventually undergo shunt placements, such as the following:
- Ventriculoperitoneal (VP) shunt (most common)
- Ventriculoatrial (VA) shunt (or "vascular shunt")
- Lumboperitoneal shunt: Only used for communicating hydrocephalus, CSF fistula, or pseudotumor cerebri)
- Torkildsen shunt (rarely): Effective only in acquired obstructive hydrocephalus
- Ventriculopleural shunt (second-line therapy): Used if other shunt types contraindicated
Rapid-onset hydrocephalus with ICP is an emergency. The following procedures can be done, depending on each specific case:
- Ventricular tap in infants
- Open ventricular drainage in children and adults
- Lumbar puncture (LP) in posthemorrhagic and postmeningitic hydrocephalus
- VP or VA shunt
Repeat LPs can be performed for cases of hydrocephalus after intraventricular hemorrhage (which can resolve spontaneously). If reabsorption does not resume when the CSF protein content is less than 100 mg/dL, spontaneous resorption is unlikely to occur. LPs can be performed only in cases of communicating hydrocephalus.
Alternatives to shunting include the following:
- Choroid plexectomy or choroid plexus coagulation
- Opening of a stenosed aqueduct
- Endoscopic fenestration of the floor of the third ventricle (however, contraindicated in communicating hydrocephalus)
Conservative management
Medical treatment is not effective in long-term treatment of chronic hydrocephalus; it is used as a temporizing measure to delay surgical intervention. Medical therapy may be tried in premature infants with posthemorrhagic hydrocephalus (in the absence of acute hydrocephalus). Normal CSF absorption may resume spontaneously during this interim period.
Medication as treatment for hydrocephalus is controversial and should be used only as a temporary measure for posthemorrhagic hydrocephalus in neonates. Such agents include carbonic anhydrase inhibitors (eg, acetazolamide) and loop diuretics (eg, furosemide).
See Treatment and Medication for more detail.
Background
Hydrocephalus can be defined broadly as a disturbance of formation, flow, or absorption of cerebrospinal fluid (CSF) that leads to an increase in volume occupied by this fluid in the CNS.[1] This condition also could be termed a hydrodynamic disorder of CSF. Acute hydrocephalus occurs over days, subacute hydrocephalus occurs over weeks, and chronic hydrocephalus occurs over months or years. Conditions such as cerebral atrophy and focal destructive lesions also lead to an abnormal increase of CSF in CNS. In these situations, loss of cerebral tissue leaves a vacant space that is filled passively with CSF. Such conditions are not the result of a hydrodynamic disorder and therefore are not classified as hydrocephalus. An older misnomer used to describe these conditions was hydrocephalus ex vacuo.
Normal pressure hydrocephalus (NPH) describes a condition that rarely occurs in patients younger than 60 years.[5] Enlarged ventricles and normal CSF pressure atlumbar puncture (LP) in the absence of papilledema led to the term NPH. However, intermittent intracranial hypertension has been noted during monitoring of patients in whom NPH is suspected, usually at night. The classic Hakim triad of symptoms includes gait apraxia, incontinence, and dementia. Headache is not a typical symptom in NPH.
Benign external hydrocephalus is a self-limiting absorption deficiency of infancy and early childhood with raised intracranial pressure (ICP) and enlarged subarachnoid spaces. The ventricles usually are not enlarged significantly, and resolution within 1 year is the rule.
Communicating hydrocephalus occurs when full communication occurs between the ventricles and subarachnoid space. It is caused by overproduction of CSF (rarely), defective absorption of CSF (most often), or venous drainage insufficiency (occasionally). See the image below.
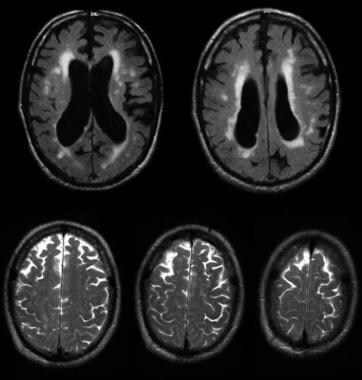 Communicating hydrocephalus with surrounding "atrophy" and increased periventricular and deep white matter signal on fluid-attenuated inversion recovery (FLAIR) sequences. Note that apical cuts (lower row) do not show enlargement of the sulci, as is expected in generalized atrophy. Pathological evaluation of this brain demonstrated hydrocephalus with no microvascular pathology corresponding with the signal abnormality (which likely reflects transependymal exudate) and normal brain weight (indicating that the sulci enlargement was due to increased subarachnoid cerebrospinal fluid [CSF] conveying a pseudoatrophic brain pattern).
Communicating hydrocephalus with surrounding "atrophy" and increased periventricular and deep white matter signal on fluid-attenuated inversion recovery (FLAIR) sequences. Note that apical cuts (lower row) do not show enlargement of the sulci, as is expected in generalized atrophy. Pathological evaluation of this brain demonstrated hydrocephalus with no microvascular pathology corresponding with the signal abnormality (which likely reflects transependymal exudate) and normal brain weight (indicating that the sulci enlargement was due to increased subarachnoid cerebrospinal fluid [CSF] conveying a pseudoatrophic brain pattern).
Noncommunicating hydrocephalus occurs when CSF flow is obstructed within the ventricular system or in its outlets to the arachnoid space, resulting in impairment of the CSF from the ventricular to the subarachnoid space. The most common form of noncommunicating hydrocephalus is obstructive and is caused by intraventricular or extraventricular mass-occupying lesions that disrupt the ventricular anatomy.[6] See the images below.
 Noncommunicating obstructive hydrocephalus caused by obstruction of the foramina of Luschka and Magendie. This MRI sagittal image demonstrates dilatation of lateral ventricles with stretching of corpus callosum and dilatation of the fourth ventricle.
Noncommunicating obstructive hydrocephalus caused by obstruction of the foramina of Luschka and Magendie. This MRI sagittal image demonstrates dilatation of lateral ventricles with stretching of corpus callosum and dilatation of the fourth ventricle.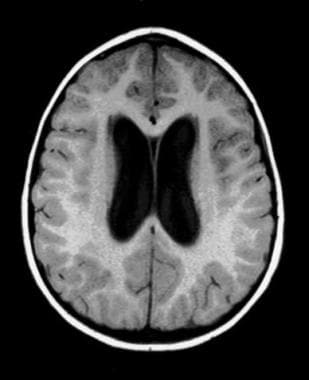 Noncommunicating obstructive hydrocephalus caused by obstruction of foramina of Luschka and Magendie. This MRI axial image demonstrates dilatation of the lateral ventricles.
Noncommunicating obstructive hydrocephalus caused by obstruction of foramina of Luschka and Magendie. This MRI axial image demonstrates dilatation of the lateral ventricles.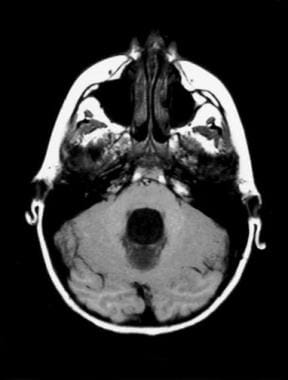 Noncommunicating obstructive hydrocephalus caused by obstruction of foramina of Luschka and Magendie. This MRI axial image demonstrates fourth ventricle dilatation.
Noncommunicating obstructive hydrocephalus caused by obstruction of foramina of Luschka and Magendie. This MRI axial image demonstrates fourth ventricle dilatation.
Congenital hydrocephalus applies to the ventriculomegaly that develops in the fetal and infancy periods, often associated with macrocephaly.[7] The most common causes of congenital hydrocephalus are obstruction of the cerebral aqueduct flow, Arnold-Chiari malformation or Dandy–Walker malformation.[8]these patients may stabilize in later years due to compensatory mechanisms but may decompensate, especially following minor head injuries. During these decompensations, determining the extent to which any new neurological deficits may be due to the new acute event, compared with hydrocephalus that may have gone unnoticed for many years, is difficult.
Pathophysiology
Normal CSF production is 0.20-0.35 mL/min; most CSF is produced by the choroid plexus, which is located within the ventricular system, mainly the lateral and fourth ventricles. The capacity of the lateral and third ventricles in a healthy person is 20 mL. Total volume of CSF in an adult is 120 mL.
Normal route of CSF from production to clearance is the following: From the choroid plexus, the CSF flows to the lateral ventricle, then to the interventricular foramen of Monro, the third ventricle, the cerebral aqueduct of Sylvius, the fourth ventricle, the 2 lateral foramina of Luschka and 1 medial foramen of Magendie, the subarachnoid space, the arachnoid granulations, the dural sinus, and finally into the venous drainage.
ICP rises if production of CSF exceeds absorption. This occurs if CSF is overproduced, resistance to CSF flow is increased, or venous sinus pressure is increased. CSF production falls as ICP rises. Compensation may occur through transventricular absorption of CSF and also by absorption along nerve root sleeves. Temporal and frontal horns dilate first, often asymmetrically. This may result in elevation of the corpus callosum, stretching or perforation of the septum pellucidum, thinning of the cerebral mantle, or enlargement of the third ventricle downward into the pituitary fossa (which may cause pituitary dysfunction).
The mechanism of NPH has not been elucidated completely. Current theories include increased resistance to flow of CSF within the ventricular system or subarachnoid villi; intermittently elevated CSF pressure, usually at night; and ventricular enlargement caused by an initial rise in CSF pressure; the enlargement is maintained despite normal pressure because of the Laplace law. Although pressure is normal, the enlarged ventricular area reflects increased force on the ventricular wall.
Frequency
United States
The incidence of congenital hydrocephalus is 3 per 1,000 live births; the incidence of acquired hydrocephalus is not known exactly due to the variety of disorders that may cause it.
International
Incidence of acquired hydrocephalus is unknown. About 100,000 shunts are implanted each year in the developed countries, but little information is available for other countries.
Mortality/Morbidity
In untreated hydrocephalus, death may occur by tonsillar herniation secondary to raised ICP with compression of the brain stem and subsequent respiratory arrest.
Shunt dependence occurs in 75% of all cases of treated hydrocephalus and in 50% of children with communicating hydrocephalus. Patients are hospitalized for scheduled shunt revisions or for treatment of shunt complications or shunt failure. Poor development of cognitive function in infants and children, or loss of cognitive function in adults, can complicate untreated hydrocephalus. It may persist after treatment. Visual loss can complicate untreated hydrocephalus and may persist after treatment.
Epidemiology
Sex
Generally, incidence is equal in males and females. The exception is Bickers-Adams syndrome, an X-linked hydrocephalus transmitted by females and manifested in males. NPH has a slight male preponderance.
Age
Incidence of human hydrocephalus presents a bimodal age curve. One peak occurs in infancy and is related to the various forms of congenital malformations. Another peak occurs in adulthood, mostly resulting from NPH. Adult hydrocephalus represents approximately 40% of total cases of hydrocephalus.
The outcome of pediatric hydrocephalus has been studied frequently, but much remains unresolved about long-term and social outcomes.[9]
History
Clinical features of hydrocephalus are influenced by the following:
- Patient's age
- Cause
- Location of obstruction
- Duration
- Rapidity of onset
Symptoms in infants include the following:
- Poor feeding
- Irritability
- Reduced activity
- Vomiting
Symptoms in children include the following:
- Slowing of mental capacity
- Headaches (initially in the morning) that are more significant than in infants because of skull rigidity
- Neck pain suggesting tonsillar herniation
- Vomiting, more significant in the morning
- Blurred vision: This is a consequence of papilledema and later of optic atrophy
- Double vision: This is related to unilateral or bilateral sixth nerve palsy
- Stunted growth and sexual maturation from third ventricle dilatation: This can lead to obesity and to precocious puberty or delayed onset of puberty.
- Difficulty in walking secondary to spasticity: This affects the lower limbs preferentially because the periventricular pyramidal tract is stretched by the hydrocephalus.
- Drowsiness
Symptoms in adults include the following:
- Cognitive deterioration: This can be confused with other types of dementia in the elderly.
- Headaches: These are more prominent in the morning because cerebrospinal fluid (CSF) is resorbed less efficiently in the recumbent position. This can be relieved by sitting up. As the condition progresses, headaches become severe and continuous. Headache is rarely if ever present in normal pressure hydrocephalus (NPH).
- Neck pain: If present, neck pain may indicate protrusion of cerebellar tonsils into the foramen magnum.
- Nausea that is not exacerbated by head movements
- Vomiting: Sometimes explosive, vomiting is more significant in the morning.
- Blurred vision (and episodes of "graying out"): These may suggest serious optic nerve compromise, which should be treated as an emergency.
- Double vision (horizontal diplopia) from sixth nerve palsy
- Difficulty in walking
- Drowsiness
- Incontinence (urinary first, fecal later if condition remains untreated): This indicates significant destruction of frontal lobes and advanced disease.
Symptoms of NPH include the following:
- Gait disturbance is usually the first symptom and may precede other symptoms by months or years. Magnetic gait is used to emphasize the tendency of the feet to remain "stuck to the floor" despite patients’ best efforts to move them.
- Dementia should be a late finding in pure (shunt-responsive) NPH. It presents as an impairment of recent memory or as a "slowing of thinking." Spontaneity and initiative are decreased. The degree can vary from patient to patient.
- Urinary incontinence may present as urgency, frequency, or a diminished awareness of the need to urinate.
- Other symptoms that can occur include personality changes and Parkinsonism. Seizures are extremely rare and should prompt consideration for an alternative diagnosis.
Physical
Physical findings in infants include the following:
- Head enlargement: Head circumference is at or above the 98th percentile for age.
- Dysjunction of sutures: This can be seen or palpated.
- Dilated scalp veins: The scalp is thin and shiny with easily visible veins.
- Tense fontanelle: The anterior fontanelle in infants who are held erect and are not crying may be excessively tense.
- Setting-sun sign: In infants, it is characteristic of increased intracranial pressure (ICP). Ocular globes are deviated downward, the upper lids are retracted, and the white sclerae may be visible above the iris.
- Increased limb tone: Spasticity preferentially affects the lower limbs. The cause is stretching of the periventricular pyramidal tract fibers by hydrocephalus.
Physical findings in children include the following:
- Papilledema: if the raised ICP is not treated, this can lead to optic atrophy and vision loss.
- Failure of upward gaze: This is due to pressure on the tectal plate through the suprapineal recess. The limitation of upward gaze is of supranuclear origin. When the pressure is severe, other elements of the dorsal midbrain syndrome (ie, Parinaud syndrome) may be observed, such as light-near dissociation, convergence-retraction nystagmus, and eyelid retraction (Collier sign).
- Macewen sign: A "cracked pot" sound is noted on percussion of the head.
- Unsteady gait: This is related to spasticity in the lower extremities.
- Large head: Sutures are closed, but chronic increased ICP will lead to progressive macrocephaly.
- Unilateral or bilateral sixth nerve palsy is secondary to increased ICP. Children with ventriculoperitoneal (VP) shunts may be more likely to have congenital esotropia.[10]
Physical findings in adults include the following:
- Papilledema: If raised ICP is not treated, it leads to optic atrophy.
- Failure of upward gaze and of accommodation indicates pressure on the tectal plate. The full Parinaud syndrome is rare.
- Unsteady gait is related to truncal and limb ataxia. Spasticity in legs also causes gait difficulty.
- Large head: The head may have been large since childhood.
- Unilateral or bilateral sixth nerve palsy is secondary to increased ICP. Children with ventricular-peritoneal shunts may be more likely to have congenital esotropia.
The following are physical findings found in NPH:
- Muscle strength is usually normal. No sensory loss is noted.
- Reflexes may be increased, and the Babinski response may be found in one or both feet. These findings should prompt search for vascular risk factors (causing associated brain microangiopathy or vascular Parkinsonism), which are common in NPH patients.
- Difficulty in walking varies from mild imbalance to inability to walk or to stand. The classic gait impairment consists of short steps, wide base, externally rotated feet, and lack of festination (hastening of cadence with progressively shortening stride length, a hallmark of the gait impairment ofParkinson disease). These abnormalities may progress to the point of apraxia. Patients may not know how to take steps despite preservation of other learned motor tasks.
- Frontal release signs such as sucking and grasping reflexes appear in late stages.
Causes
Congenital causes in infants and children include the following:[7]
- Brainstem malformation causing stenosis of the aqueduct of Sylvius: This is responsible for 10% of all cases of hydrocephalus in newborns.
- Dandy-Walker malformation: This affects 2-4% of newborns with hydrocephalus.
- Arnold-Chiari malformation type 1 and type 2
- Agenesis of the foramen of Monro
- Congenital toxoplasmosis
- Bickers-Adams syndrome: This is an X-linked hydrocephalus accounting for 7% of cases in males. It is characterized by stenosis of the aqueduct of Sylvius, severe mental retardation, and in 50% by an adduction-flexion deformity of the thumb.
Acquired causes in infants and children include the following:
- Mass lesions: Mass lesions account for 20% of all cases of hydrocephalus in children. These are usually tumors (eg, medulloblastoma, astrocytoma), but cysts, abscesses, or hematoma also can be the cause.[11]
- Hemorrhage: Intraventricular hemorrhage can be related to prematurity, head injury, or rupture of a vascular malformation.
- Infections: Meningitis (especially bacterial) and, in some geographic areas,cysticercosis can cause hydrocephalus.
- Increased venous sinus pressure: This can be related to achondroplasia, some craniostenoses, or venous thrombosis.
- Iatrogenic: Hypervitaminosis A, by increasing secretion of CSF or by increasing permeability of the blood-brain barrier, can lead to hydrocephalus. As a caveat, hypervitaminosis A is a more common cause of idiopathic intracranial hypertension, a disorder with increased CSF pressure but small rather than large ventricles.
- Idiopathic
Causes of hydrocephalus in adults include:
- Subarachnoid hemorrhage (SAH) causes one third of these cases by blocking the arachnoid villi and limiting resorption of CSF. However, communication between ventricles and subarachnoid space is preserved.[12]
- Idiopathic hydrocephalus represents one third of cases of adult hydrocephalus.
- Head injury, through the same mechanism as SAH, can result in hydrocephalus.
- Tumors can cause blockage anywhere along the CSF pathways. The most frequent tumors associated with hydrocephalus are ependymoma, subependymal giant cell astrocytoma, choroid plexus papilloma, craniopharyngioma, pituitary adenoma, hypothalamic or optic nerve glioma, hamartoma, and metastatic tumors.
- Prior posterior fossa surgery may cause hydrocephalus by blocking normal pathways of CSF flow.
- Congenital aqueductal stenosis causes hydrocephalus but may not be symptomatic until adulthood. Special care should be taken when attributing new neurological deficits to congenital hydrocephalus, as its treatment by shunting may not correct these deficits.
- Meningitis, especially bacterial, may cause hydrocephalus in adults.
- All causes of hydrocephalus described in infants and children are present in adults who have had congenital or childhood-acquired hydrocephalus.
Causes of NPH may include the following (Most cases are idiopathic and are probably related to a deficiency of arachnoid granulations.):
- SAH
- Head trauma
- Meningitis
Laboratory Studies
No specific blood tests are recommended in the workup for hydrocephalus.Genetic testing and counseling might be recommended when X-linked hydrocephalus is suspected.Evaluate cerebrospinal fluid (CSF) in posthemorrhagic and postmeningitic hydrocephalus for protein concentration and to exclude residual infection.Imaging Studies
CT can assess the size of ventricles and other structures.MRI can evaluate for Chiari malformation or cerebellar or periaqueductal tumors. It affords better imaging of the posterior fossa than CT. MRI can differentiate normal pressure hydrocephalus (NPH) from cerebral atrophy although the distinctions may be challenging. Flow voids in the third ventricle and transependymal fluid exudates are helpful. However, numerous suitable patients have a brain pattern suggestive of atrophy and small vessel ischemic disease that may ultimately be NPH.[13] Guidelines for imaging studies in suspected NPH have been established.[14]CT/MRI criteria for acute hydrocephalus include the following:- Size of both temporal horns is greater than 2 mm, clearly visible. In the absence of hydrocephalus, the temporal horns should be barely visible.
- Ratio of the largest width of the frontal horns to maximal biparietal diameter (ie, Evans ratio) is greater than 30% in hydrocephalus.
- Transependymal exudate is translated on images as periventricular hypoattenuation (CT) or hyperintensity (MRI T2-weighted and fluid-attenuated inversion recovery [FLAIR] sequences).
- Ballooning of frontal horns of lateral ventricles and third ventricle (ie, "Mickey mouse" ventricles) may indicate aqueductal obstruction.
- Upward bowing of the corpus callosum on sagittal MRI suggests acute hydrocephalus.
CT/MRI criteria for chronic hydrocephalus include the following:- Temporal horns may be less prominent than in acute hydrocephalus.
- Third ventricle may herniate into the sella turcica.
- Sella turcica may be eroded.
- Macrocrania (ie, occipitofrontal circumference >98 th percentile) may be present.
- Corpus callosum may be atrophied (best appreciated on sagittal MRI). In this case, parenchymal atrophy and ex-vacuo (rather than true) hydrocephalus from a neurodegenerative disease should be considered.
Ultrasonography through the anterior fontanelle in infants is useful for evaluating subependymal and intraventricular hemorrhage and in following infants for possible development of progressive hydrocephalus.Radionuclide cisternography can be done in NPH to evaluate the prognosis with regard to possible shunting. If a late scan (48-72 h) shows persistence of ventricular activity with a ventricular to total intracranial activity (V/T ratio) greater than 32%, the patient is more likely to benefit from shunting.[15] Because of its poor sensitivity in predicting shunt response when the V/T ration is less than 32%, this test is no longer commonly used.Skull radiographs may depict erosion of sella turcica, or "beaten copper cranium" (called by some authors "beaten silver cranium"). The latter can also be seen in craniosynostosis.MRI cine is an MRI technique to measure CSF stroke volume (SV) in the cerebral aqueduct. Cine phase-contrast MRI measurements of SV in the cerebral aqueduct does not appear to be useful in predicting response to shunting.[2]Diffusion tensor imaging (DTI) is a novel imaging technique that detects differences in fractional anisotropy (FA) and mean diffusivity (MD) of the brain parenchyma surrounding the ventricles. Impairment of FA and MD through DTI allows the recognition of microstructural changes in periventricular white matter region that may be too subtle on conventional MRI.[3]Other Tests
See the list below:- After shunt insertion, confirm correct positioning of installed hardware with a plain radiograph.
- EEG if seizure occurs
Procedures
Lumbar puncture (LP) is a valuable test in evaluating NPH, but should be performed only after CT or MRI of the head. Normal LP opening pressure (OP) should be less than 180 mm H2 O (ie, 18 cm H2 O). Patients with initial OP greater than 100 mm H2 O have a higher rate of response to CSF shunting than those with OPs less than 100 mm H2 O. Improvement of symptoms after a single LP in which 40-50 mL of CSF is withdrawn appears to have some predictive value for success of CSF shunting.Continuous CSF drainage through external lumbar drainage (ELD) is a highly accurate test for predicting the outcome after ventricular shunting in NPH, although false negative results are not uncommon.[16]Continuous CSF pressure monitoring can help in predicting a patient's response to CSF shunting in NPH. Some patients with normal OP on LP demonstrate pressure peaks of greater than 270 mm H2 O or recurrent B waves. These patients tend to have higher rates of response to shunting than those who do not have these findings. This procedure also could differentiate NPH from atrophy.Histologic Findings
Histologic findings include the following:- Thinning and stretching of the cortical mantle may be seen as a result of ventricular dilation.
- In the acute phase, edema of the periventricular white matter is observed. Relatively few neuronal lesions are present. Ventricular ependyma shows cellular flattening and loss of cilia.
- At a later stage, the edema disappears and is replaced by fibrosis, axonal degeneration, demyelination, focal loss of cerebral cortical neurons, cellular flattening, and further loss of cilia.
Medical Care
Medical treatment in hydrocephalus is used to delay surgical intervention. It may be tried in premature infants with posthemorrhagic hydrocephalus (in the absence of acute hydrocephalus). Normal CSF absorption may resume spontaneously during this interim period.Medical treatment is not effective in long-term treatment of chronic hydrocephalus. It may induce metabolic consequences and thus should be used only as a temporizing measure.Medications affect CSF dynamics by the following mechanisms:- Decreasing CSF secretion by the choroid plexus - Acetazolamide and furosemide
- Increasing CSF reabsorption - Isosorbide (effectiveness is questionable)
Surgical Care
Surgical treatment is the preferred therapeutic option.[4]Repeat lumbar punctures (LPs) can be performed for cases of hydrocephalus after intraventricular hemorrhage, since this condition can resolve spontaneously. If reabsorption does not resume when the protein content of cerebrospinal fluid (CSF) is less than 100 mg/dL, spontaneous resorption is unlikely to occur. LPs can be performed only in cases of communicating hydrocephalus.Alternatives to shunting include the following:- Choroid plexectomy or choroid plexus coagulation may be effective.
- Opening of a stenosed aqueduct has a higher morbidity rate and a lower success rate than shunting, except in the case of tumors. However, lately cerebral aqueductoplasty has gained popularity as an effective treatment for membranous and short-segment stenoses of the sylvian aqueduct. It can be performed through a coronal approach or endoscopically through suboccipital foramen magnum trans-fourth ventricle approach.
- In these cases, tumor removal cures the hydrocephalus in 80%.
- Endoscopic fenestration of the floor of the third ventricle establishes an alternative route for CSF toward the subarachnoid space. It is contraindicated in communicating hydrocephalus.
Shunts eventually are performed in most patients. Only about 25% of patients with hydrocephalus are treated successfully without shunt placement. The principle of shunting is to establish a communication between the CSF (ventricular or lumbar) and a drainage cavity (peritoneum, right atrium, pleura). Remember that shunts are not perfect and that all alternatives to shunting should be considered first.- A ventriculoperitoneal (VP) shunt is used most commonly. The lateral ventricle is the usual proximal location. The advantage of this shunt is that the need to lengthen the catheter with growth may be obviated by using a long peritoneal catheter.
- A ventriculoatrial (VA) shunt also is called a "vascular shunt." It shunts the cerebral ventricles through the jugular vein and superior vena cava into the right cardiac atrium. It is used when the patient has abdominal abnormalities (eg, peritonitis, morbid obesity, or after extensive abdominal surgery). This shunt requires repeated lengthening in a growing child.
- A lumboperitoneal shunt is used only for communicating hydrocephalus, CSF fistula, or pseudotumor cerebri.
- A Torkildsen shunt is used rarely. It shunts the ventricle to cisternal space and is effective only in acquired obstructive hydrocephalus.
- A ventriculopleural shunt is considered second line. It is used if other shunt types are contraindicated.
Rapid-onset hydrocephalus with increased intracranial pressure (ICP) is an emergency. The following can be done, depending on each specific case:- Ventricular tap in infants
- Open ventricular drainage in children and adults
- LP in posthemorrhagic and postmeningitic hydrocephalus
- VP or VA shunt
Consultations
Consultation with the following may prove helpful:- Neurosurgeon
- Neurologist
- Neurorehabilitation specialist
- Ophthalmologist
Activity
Most surgeons agree that, with the use of antisiphon devices, no special positioning is required after shunting. However, some surgeons used to leave patients in whom a standard shunt had been placed in a recumbent position for 1-2 days after surgery to minimize risk of subdural hematoma.In treatment of normal pressure hydrocephalus (NPH), gradual postoperative mobilization is recommended.






0 التعليقات:
Post a Comment